Teeth Whitening: Does it work and is it safe?
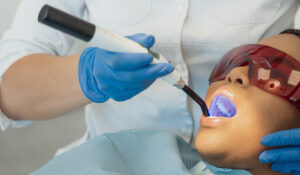
A beautiful smile and good oral health are frequently linked. Teeth Whitening in San Antonio, TX, has become a common aesthetic dentistry procedure. There
14500 San Pedro Ave #200, San Antonio, TX 78232, United States

A beautiful smile and good oral health are frequently linked. Teeth Whitening in San Antonio, TX, has become a common aesthetic dentistry procedure. There

A root canal is a dental operation that can keep a tooth from being extracted if it is severely decayed or diseased. Proper aftercare
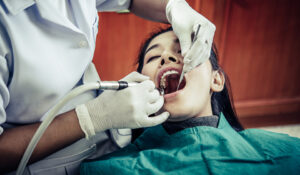
A root canal, commonly referred to as root canal therapy, is a dental procedure that can keep a damaged or infected tooth from needing

Monday – 9:00 AM – 4:00 PM
Tuesday – 9:00 AM – 4:00 PM
Wednesday – 9:00 AM – 4:00 PM
Copyright © 2023 Mike Majors D.D.S. All rights reserved.